Which Best Describes the Ions in a Neutrsl Solution
H acceptor B basic. A pH value is used to describe a water-based solution.

Neutral Solution Definition Examples Video Lesson Transcript Study Com
The following shows the various confirmatory tests for carbonate ion chloride ion sulphate ion and nitrate ion in aqueous solutions.

. Note that this MOLES of H or OH- ions per litre of water. There is also 10-7 mol OH- ions per litre of water. 1 mol H ions 60221023 ions.
This corresponds to a concentration of 10-7 mol H ions per litre of water. Positive ions have more protons than neutrons. H donor D basic.
PH of more than 7 is basic. A buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating hydrogen ions when bases are added. Pure water autoionizes only sparingly so Kw is small.
Which of the following best describes this solution. When a salt is dissolved in water the free anion will be present in the aqueous solution. Positive ions have more electrons than neutrons.
The Cl ion is the conjugate base of the strong acid HCl so it has essentially no basic character. Tests can then be carried out to identify the anion. H acceptor B basic.
A solution contains 00000001 10-7 moles of hydroxyl ions OH- per liter. How does the addition of an acid affect a neutral solution the h ion concentration is increased a given solution contains 000011 10-4 moles of hydrogen ions h per liter. View solution Which of the following statement is not correct for the element having electronic configuration 1 s 2 2 s.
The excess protons push the remaining electrons away. D Buffered solutions are always neutral with a pH of 7. Up to 256 cash back C Buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating hydrogen ions when bases are added A buffer stabilizes the pH of a solution by preventing acids or bases from dissociating.
H acceptor C acidic. The remaining electrons are more easily lost. Which of the following best describes this solution.
A solution contains 10-3 moles of hydroxyl ions OH- per liter. Which of the following best describes this solution. In general a small pH value describes a solution that is acidic and a larger pH value describing solutions that are less acidic more basicThe pH scale is centered on 7 - meaning that a solution with a pH of 7 is perfectly neutral neither acidic nor basic.
Test for anions in aqueous solutions. The excess protons pull harder on the fewer electrons O C. H donor D neutral Answer.
Which best describes what happens when a neutral atom becomes a positive ion. Polar QUESTION 3 A solution contains 0000000110-7 moles of hydroxyl ions OH- per liter. H acceptor B acidic.
The autoionization constant of water cannot be used for interconverting between concentrations of hydroxide and hydronium ions. H acceptor C acidic. Now lets compare this behavior to the behavior of aqueous solutions of potassium cyanide and sodium acetate.
An antacid decreases the level of hydrogen ions. Neutral solutions maintain a pH of 7. 32 A solution contains 00000001 10-7 moles of hydroxyl ions OH- per liter.
The best term to describe this mixture would be _____ an aqueous solution If the molecular mass of a carbon atom is 12 the mass of a hydrogen atom is 1 and the mass of an oxygen atom is 16 daltons how many molecules does one mole of table sugar sucrose. Numbers from 0-7 are considered acidic. Click Save and Submit to save and submit.
H acceptor C acidic. 3 Flashcards Quizlet Select the statement that best describes a buffer. Which of the following best describes this solution.
An antacid neutralizes excess hydrogen ions. PH of less than 7 is acidic. H donor D neutral.
Which of the following best describes this solution. A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. A buffer stabilizes the pH of a.
Which of the following choices best describes the auto-ionization constant of water. Vinegar fruit juice and cola are examples of. The pH of a solution indicates the general level of reactivity based upon whether the substance is acidic or basic.
K w OH-H 3 O Group of answer choices. H donor E neutral. Water and human blood are great examples of neutral solutions.
Which of the following best describes this solution. Pure water autoionizes readily so Kw is large. The remaining electrons spread farther out.
What do hydrogen ions. An antacid increases the amount of hydrogen ions. A solution contains 00000001 10-7 moles of hydrogen ions H per liter.
H acceptor QUESTION 4 In pure water H and OH- concentrations are equal. 11218 958 PM MB Chp. Numbers from 7-14 are considered basic.
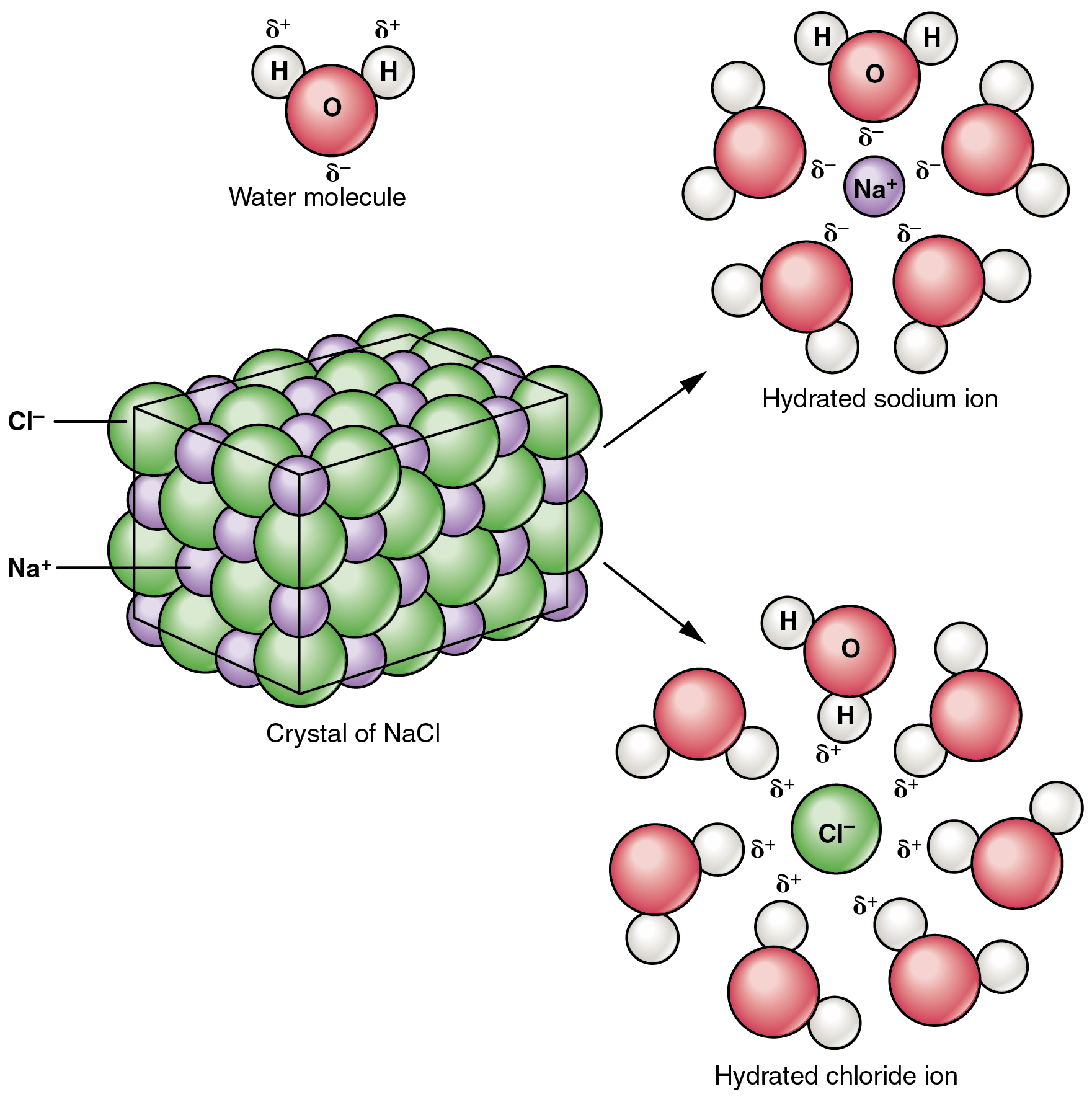
Consequently dissolving NaCl in water has no effect on the pH of a solution and the solution remains neutral. If you want to know the actual number of H ions in the litre of water. Which statement describes positive ions.
Which statement best describes an antacid. Test for carbonate ion CO 3.

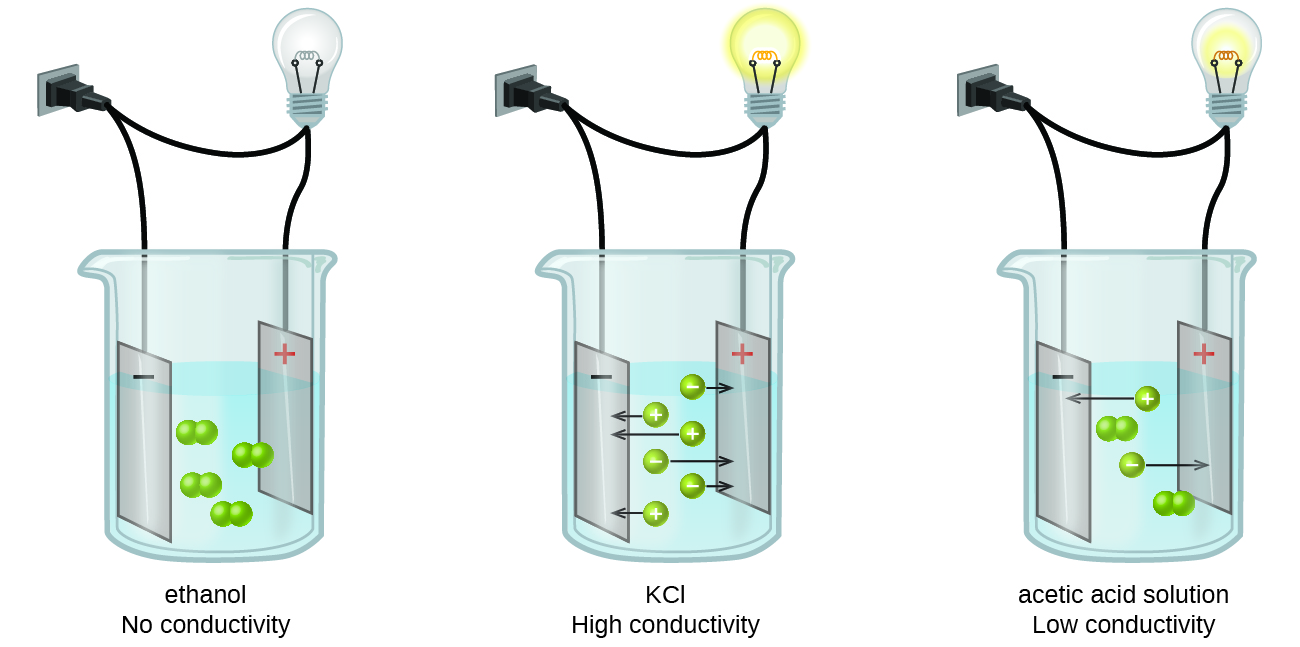
Physical Properties Of Ionic Compounds Chemistry For Non Majors
Molecular Complete Ionic And Net Ionic Equations Article Khan Academy
Comments
Post a Comment